Is there any impact of COVID-19 on cord blood and tissue stem cell collection and storage?
As the potential consequences of the coronavirus (COVID-19) are felt across many aspects of our lives, we’re reaching out to update our client families. We want to anticipate potential questions regarding the impact of the virus on cord blood collection, storage and therapeutic use.
Cell Care recognises that banking a child’s umbilical cord stem cells is a once-in-a-lifetime opportunity for a family - they may only be collected at birth. Many parents are emphasising the importance of investing in future health care options for their family. We're maintaining our laboratory and collection network in full operation to ensure uninterrupted services. Our client services team can help you or your friend referrals with any questions on 1800 071 075 .
Umbilical cord stem cells already cryo-preserved with Cell Care are not, and cannot be, impacted in any way by COVID-19
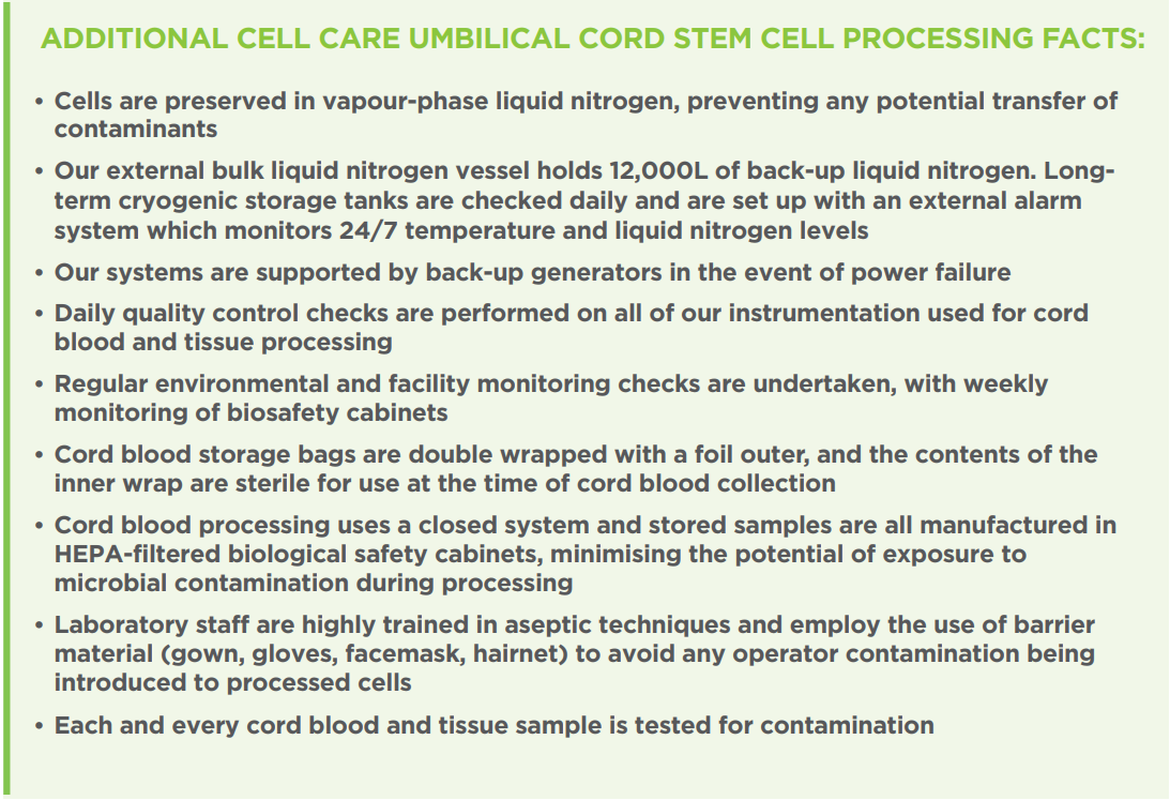
Our storage system preserves cells in vapour-phase liquid nitrogen at -196 degrees Celsius – preventing any possible transfer of any contaminant. All 150,000+ samples cryogenically stored by the Cell Care group internationally are stored under these conditions. Ongoing laboratory validations that we undertake consistently reaffirm this fact. Our laboratory processing methodologies adhere to best in class Good Manufacturing Processes (GMP). Government and Regulatory audits of our closed system processing highlight our product quality. We are one of the most accredited cord blood banking groups in the world. Across our group, we sustain FACT and AABB accreditation, and operate under TGA and Health Canada licences.
If an expectant mother is COVID-19 infected, is there a risk it can pass to the newborn via the cord blood?
There is no evidence that COVID-19 can be transmitted through cord blood, nor is there evidence of intrauterine vertical transmission from the mother. Since cord blood collections require thorough screening of the mother (including medical history), the chance of any transmission via cord blood is considered extremely low to non-existent.
(Source: Qiao J et al (The Lancet 395); Chen H et al (The Lancet 395); MedPage ed. Feb 12 (Walker M))
Can COVID-19 be transferred into a recipient patient following cord blood transplantation/infusion?
Blood cells lack the binding sites required by the coronavirus to bind and replicate. The virus is focused on attacking the respiratory and digestive systems. Like the SARS virus, COVID-19 must enter a cell before it can start replicating. Access points for COVID-19 are indeed expressed almost all over the human body, but at high levels in the lungs and intestines, and in the oral and nasal mucosa and tongue.
Is Cell Care testing cord blood or maternal blood samples for COVID-19?
Cell Care is not currently testing blood samples for COVID-19. Our Medical Questionnaire, completed by mothers prior to cord blood collection is being updated to request information related to the infection, including recent travel patterns and potential close contact with someone diagnosed with COVID-19. The responses will determine the requirement for follow up testing.
NB: Blood cells (maternal, umbilical) lack the binding sites required by the coronavirus to bind and replicate.
What is COVID-19?
COVID-19 is a relatively highly infectious disease caused by the most recently discovered coronavirus. The disease can spread from person to person through small respiratory droplets which are spread when a person with COVID-19 coughs or exhales. Other people then catch COVID-19 by breathing in these droplets or by touching surfaces where the droplets have landed then touching their eyes, nose or mouth.
(source: https://www.who.int/news-room/q-a- detail/q-a-coronaviruses).
While the COVID -19 situation continues to evolve, we are doing everything possible to maintain business as usual. If you have further questions, do not hesitate to contact our Client Services team on 1800 071 075 .